About SWAN
SWAN examines the physical, biological, psychological and social changes during this transitional period. The goal of SWAN’s research is to help scientists, health care providers and women learn how mid-life experiences affect health and quality of life during aging.
The study is co-sponsored by the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), the National Institutes of Health (NIH), Office of Research on Women’s Health, and the National Center for Complementary and Alternative Medicine.
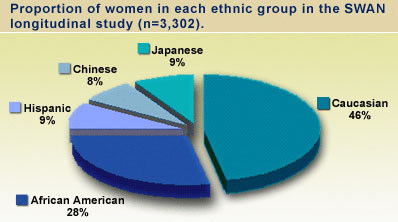
The study began in 1994. Between 1996 and 1997, 3,302 participants joined SWAN through seven designated research centers. The research centers are located in the following communities: Ann Arbor, MI (University of Michigan), Boston, MA (Massachusetts General Hospital), Chicago, IL (Rush University Medical Center), Alameda and Contra Costa County, CA (University of California Davis and Kaiser Permanente), Los Angeles, CA (University of California at Los Angeles), Jersey City, NJ (Albert Einstein College of Medicine), and Pittsburgh, PA (University of Pittsburgh). SWAN participants represent five racial/ethnic groups and a variety of backgrounds and cultures.
The NIA Biobank, the biologic specimen bank for SWAN, contains blood and urine specimens collected at each study participant’s annual visit.
Additional information can be obtained from NIA’s Public Information Office at (301) 496-1752.

Funding Agencies:
- National Institute on Aging
- National Institute of Nursing Research
- National Institute of Child Health & Human Development
- National Institute of Mental Health
- Office of Research on Women’s Health
- National Center for Complementary and Alternative Medicine
- Department of Defense Congressionally Directed Medical Research Programs
SWAN History
In 1994, SWAN was designed as a multi-site, observational study to be conducted in three phases. The initial phase consisted of focus groups of women with characteristics similar to those subsequently enrolled in SWAN. These focus groups were conducted in order to develop the most effective and culturally sensitive study design and protocols for recruiting and retaining diverse groups of women into SWAN.

The second phase was the cross-sectional survey, a 15-minute interview, which was administered to women who met the following criteria: residence within the geographic area specified by the clinical site; ability to speak and read English or the designated language (Chinese, Japanese or Spanish) of the clinical site, age 40-55; and self-identification within one of the two race/ethnicity groups studied at the clinical site. Each site used population survey methods to contact a generally representative group of women for the cross-sectional telephone interview. Interviews were conducted by telephone or in person. The SWAN cross-sectional screener dataset has been archived at the National Archive of Computerized Data on Aging (NACDA). To find out more go to the NACDA website, click on search holdings and type in SWAN.
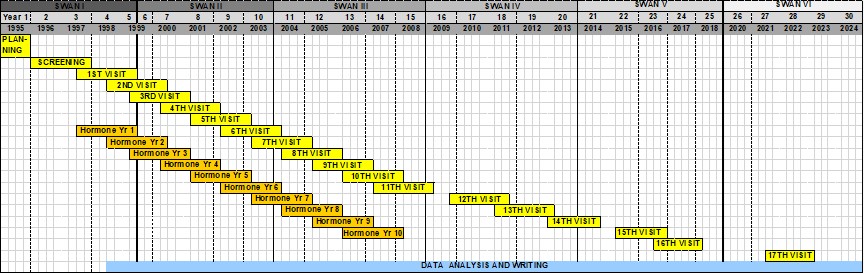
In the third phase, eligible premenopausal women were enrolled from the cross-sectional phase into the longitudinal follow-up study. Women who met the eligibility criteria were 42-52 years old, had a uterus and at least one intact ovary, reported a menstrual period within the past three months and had not taken hormone medications (such as birth control pills, estrogen or progesterone preparations) in the last three months. Enrollment into this phase began in January 1996 and all seven clinical sites completed the baseline visit by December 1997. A total of 3,302 eligible women were enrolled into the longitudinal study population of SWAN and completed the baseline study (1,550 Caucasian, 935 African American, 286 Hispanic, 250 Chinese, and 281 Japanese).
SWAN has completed the screening, baseline and 16 follow-up visits. Visits have taken place approximately every year. The annual visit has included the following core components: physical measures (weight, height, hip, waist, and blood pressure), fasting morning blood draw, interviewer-administered and self-administered questionnaires. Women were also given menstrual calendars to complete monthly over the next year. All questionnaires were translated into Spanish, Cantonese, and Japanese and could be administered by bilingual interviewers.
SWAN is currently in its sixth funding period, which includes a single in-person visit (visit 17) for the whole cohort. In addition to the annual visit core components listed above, new measures of cognition, sleep, physical activity, cardiovascular health, social functioning, and urogenital and sexual health are being completed by SWAN participants at visit 17.